The crystal structure of NTC thermistor
The main crystal phase of Ni-Mn-O system NTC thermal ceramics is generally cubic spinel structure. The structure is cubic crystal system, the space group symbol is Fd3m, and its crystallographic general formula is AB2O4. In this structure, oxygen ions O2- are densely packed in space in a cubic form, resulting in two interstitial positions, namely, octahedral interstices and tetrahedral interstitials, and cations occupy these two interstitial positions. If a cation enters the tetrahedral interstitial position (A position), it is called the 8a position in crystallography, and its spatial coordinate parameters are (1/4, 1/4, 1/4). If the cation enters the octahedral interstitial position (B position), it is called the 16d position in crystallography. Its spatial coordinate parameter is (3/8, 5/8, 3/8), the coordinate parameter of O2-ion is (u, u, u), and its crystallographic position is 32e. The specific spinel spatial structure is shown in Figure 1.
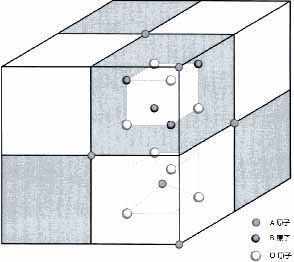
The distribution of cations in the two interstitial positions in the spinel structure directly determines the electrical properties of NTC thermal ceramics. The distribution of cations in the spinel structure is closely related to many factors. These influencing factors can be roughly divided into two categories: The first category is the intrinsic factors related to the cation itself, such as the extranuclear electronic configuration, radius and valence of the cation; The second category is extrinsic factors related to external process conditions, such as cooling rate, temperature and atmosphere. Among them, the first type of intrinsic influencing factors is the most important influencing factor, so we mainly discuss the influence of this type of factors on the distribution of cations. The first type of intrinsic influencing factors mainly include the neutral rule of electricity price, the size of the ionic radius, and the configuration of extranuclear electrons. First, discuss neutral rules for electricity prices. The endpoints of each tetrahedron in the spinel structure are shared with the three adjacent octahedrons, and the octahedron shares six edges with the adjacent octahedrons. This means that each octahedral vertex is shared with the adjacent 2 octahedrons and 1 tetrahedron, and each O2-ion has 3 B-site cations and 1 A-site cation around it. For spinel of type 2:3 (that is, the cation at the A position is +2 valence, and the cation at the B position is +3 valence), if the cations are distributed in the structure of the normal spinel, it is just in line with the neutral electricity price rule: (1×2/ 4+3×3/6)=2(O2 -); For 4:2 (A-site cation + 4 valence, B site + 2 valence) spinel. If the positive spinel structure continues to be maintained, the neutral price rule will not be met at this time: (1×2/ 4+3×4/6)≠2. In order to resolve this contradiction, if the 4:2 type spinel adopts the reverse type spinel cation distribution, it just conforms to the neutral rule of electricity price: [(1/2)×3×(4/6)+(1/2)×3×(2/6)+1×2/4]=2. From the perspective of the neutral rule of electricity price, the 2:3 type spinel cation distribution tends to the normal spinel structure, and the 4:2 type spinel cation distribution tends to the inverse spinel structure.
Secondly, discuss the influence of ion radius. The radius of +2-valent cations is generally larger than that of +3-valent cations, so +2-valent cations tend to enter the octahedral gap with larger space. The +3 valent cation tends to enter the tetrahedral gap with smaller space. Considering only the ionic radius, the cation distribution of the 2:3 type spinel tends to the inverse spinel structure, and the 4:2 type spinel cation distribution tends to the normal spinel structure. It can be seen that the two factors of cation valence and ionic radius have opposite effects on the distribution of cations in the spinel structure, and they can basically cancel each other out. Therefore, the extranuclear electronic configuration of the cation becomes more important.
In crystal fields with different coordination numbers, cations with different external electron configurations have crystal field stabilization energy (CFSE) with different energies. The theory of crystal field coordination points out: In the crystal field, the external electron configuration of the cation will undergo energy level splitting, and the filling of electrons into the split energy level will cause additional energy reduction, which is beneficial to the stability of the crystal field. The cations have different crystal field stabilization energies in the octahedral position and the tetrahedral position in the spinel structure. The stability energies of some common 3d transition metal ions in the octahedral and tetrahedral crystal field and the preferential occupation energy (OSPE) in the octahedral field are shown in Table 1. It can be seen from Table 1: The CFSE of Ni2+ ions in the tetrahedral field is only 6.5, while the CFSE in the octahedral field is 29.3, so the OSPE of Ni2+ ions in the octahedral interstitial position is 22.8. This means that Ni2+ ions preferentially enter the octahedral position, which is the B site in the spinel structure.
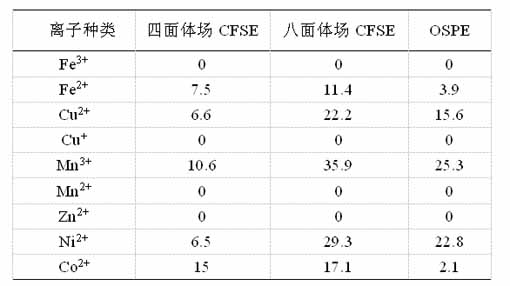
Table 1. The crystal field stabilization energy (CFSE) of some common 3d transition metal cations in the octahedral and tetrahedral crystal field, and the preferential occupation energy in the octahedral field
In addition, some extrinsic factors related to external process conditions, such as cooling rate, sintering temperature, etc., will also affect the distribution of cations in the spinel structure. It is generally believed that the higher the sintering temperature (below the decomposition temperature), the more the cations are distributed in disorder. This is because the entropy increases at high temperatures, which leads to increased chaos, and the cation distribution becomes more disordered. The faster the cooling rate is, the more the proportion of the retained cation distribution component is frozen at high temperature. With the help of neutron diffraction method, Baudour and other researchers characterized the cation distribution of Ni0.8Mn2.2O4 samples after different cooling speeds: After the sample is sintered at 1160°C, the temperature is cooled at a rate of -5°C/min. The distribution of cations is as follows: Ni2+0.02Mn3+0.09Mn2+0.89[Ni2+0.78Mn4+0.69Mn3+0.53]O4; When quenching at 900°C for rapid cooling, the distribution of cations is as follows: Ni2+0.087Mn2+0.913 [Ni2+0.713Mn4+0.713 Mn3+0.574]O4. It can be seen that the proportion of Ni2+ ions that are frozen and retained at the A site will increase when the cooling rate is faster.

The distribution of cations in the two interstitial positions in the spinel structure directly determines the electrical properties of NTC thermal ceramics. The distribution of cations in the spinel structure is closely related to many factors. These influencing factors can be roughly divided into two categories: The first category is the intrinsic factors related to the cation itself, such as the extranuclear electronic configuration, radius and valence of the cation; The second category is extrinsic factors related to external process conditions, such as cooling rate, temperature and atmosphere. Among them, the first type of intrinsic influencing factors is the most important influencing factor, so we mainly discuss the influence of this type of factors on the distribution of cations. The first type of intrinsic influencing factors mainly include the neutral rule of electricity price, the size of the ionic radius, and the configuration of extranuclear electrons. First, discuss neutral rules for electricity prices. The endpoints of each tetrahedron in the spinel structure are shared with the three adjacent octahedrons, and the octahedron shares six edges with the adjacent octahedrons. This means that each octahedral vertex is shared with the adjacent 2 octahedrons and 1 tetrahedron, and each O2-ion has 3 B-site cations and 1 A-site cation around it. For spinel of type 2:3 (that is, the cation at the A position is +2 valence, and the cation at the B position is +3 valence), if the cations are distributed in the structure of the normal spinel, it is just in line with the neutral electricity price rule: (1×2/ 4+3×3/6)=2(O2 -); For 4:2 (A-site cation + 4 valence, B site + 2 valence) spinel. If the positive spinel structure continues to be maintained, the neutral price rule will not be met at this time: (1×2/ 4+3×4/6)≠2. In order to resolve this contradiction, if the 4:2 type spinel adopts the reverse type spinel cation distribution, it just conforms to the neutral rule of electricity price: [(1/2)×3×(4/6)+(1/2)×3×(2/6)+1×2/4]=2. From the perspective of the neutral rule of electricity price, the 2:3 type spinel cation distribution tends to the normal spinel structure, and the 4:2 type spinel cation distribution tends to the inverse spinel structure.
Secondly, discuss the influence of ion radius. The radius of +2-valent cations is generally larger than that of +3-valent cations, so +2-valent cations tend to enter the octahedral gap with larger space. The +3 valent cation tends to enter the tetrahedral gap with smaller space. Considering only the ionic radius, the cation distribution of the 2:3 type spinel tends to the inverse spinel structure, and the 4:2 type spinel cation distribution tends to the normal spinel structure. It can be seen that the two factors of cation valence and ionic radius have opposite effects on the distribution of cations in the spinel structure, and they can basically cancel each other out. Therefore, the extranuclear electronic configuration of the cation becomes more important.
In crystal fields with different coordination numbers, cations with different external electron configurations have crystal field stabilization energy (CFSE) with different energies. The theory of crystal field coordination points out: In the crystal field, the external electron configuration of the cation will undergo energy level splitting, and the filling of electrons into the split energy level will cause additional energy reduction, which is beneficial to the stability of the crystal field. The cations have different crystal field stabilization energies in the octahedral position and the tetrahedral position in the spinel structure. The stability energies of some common 3d transition metal ions in the octahedral and tetrahedral crystal field and the preferential occupation energy (OSPE) in the octahedral field are shown in Table 1. It can be seen from Table 1: The CFSE of Ni2+ ions in the tetrahedral field is only 6.5, while the CFSE in the octahedral field is 29.3, so the OSPE of Ni2+ ions in the octahedral interstitial position is 22.8. This means that Ni2+ ions preferentially enter the octahedral position, which is the B site in the spinel structure.

Table 1. The crystal field stabilization energy (CFSE) of some common 3d transition metal cations in the octahedral and tetrahedral crystal field, and the preferential occupation energy in the octahedral field
In addition, some extrinsic factors related to external process conditions, such as cooling rate, sintering temperature, etc., will also affect the distribution of cations in the spinel structure. It is generally believed that the higher the sintering temperature (below the decomposition temperature), the more the cations are distributed in disorder. This is because the entropy increases at high temperatures, which leads to increased chaos, and the cation distribution becomes more disordered. The faster the cooling rate is, the more the proportion of the retained cation distribution component is frozen at high temperature. With the help of neutron diffraction method, Baudour and other researchers characterized the cation distribution of Ni0.8Mn2.2O4 samples after different cooling speeds: After the sample is sintered at 1160°C, the temperature is cooled at a rate of -5°C/min. The distribution of cations is as follows: Ni2+0.02Mn3+0.09Mn2+0.89[Ni2+0.78Mn4+0.69Mn3+0.53]O4; When quenching at 900°C for rapid cooling, the distribution of cations is as follows: Ni2+0.087Mn2+0.913 [Ni2+0.713Mn4+0.713 Mn3+0.574]O4. It can be seen that the proportion of Ni2+ ions that are frozen and retained at the A site will increase when the cooling rate is faster.





